f block Elements Lanthanides and Actinides Periodic Table
Steps for Identifying S, P, D, & F-Block Elements. Step 1: Find the element on the periodic table. Step 2: Use periodic table landmarks and mnemonic devices to determine the block. Vocabulary for.
PPT Chapter 8 PowerPoint Presentation, free download ID6535306
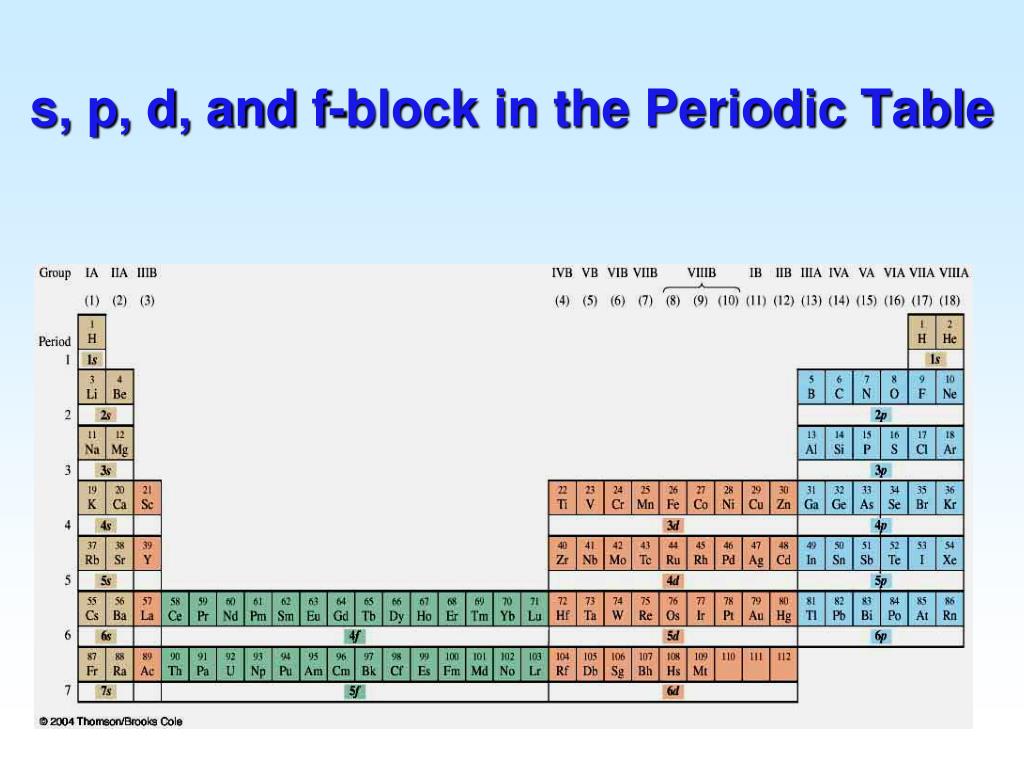
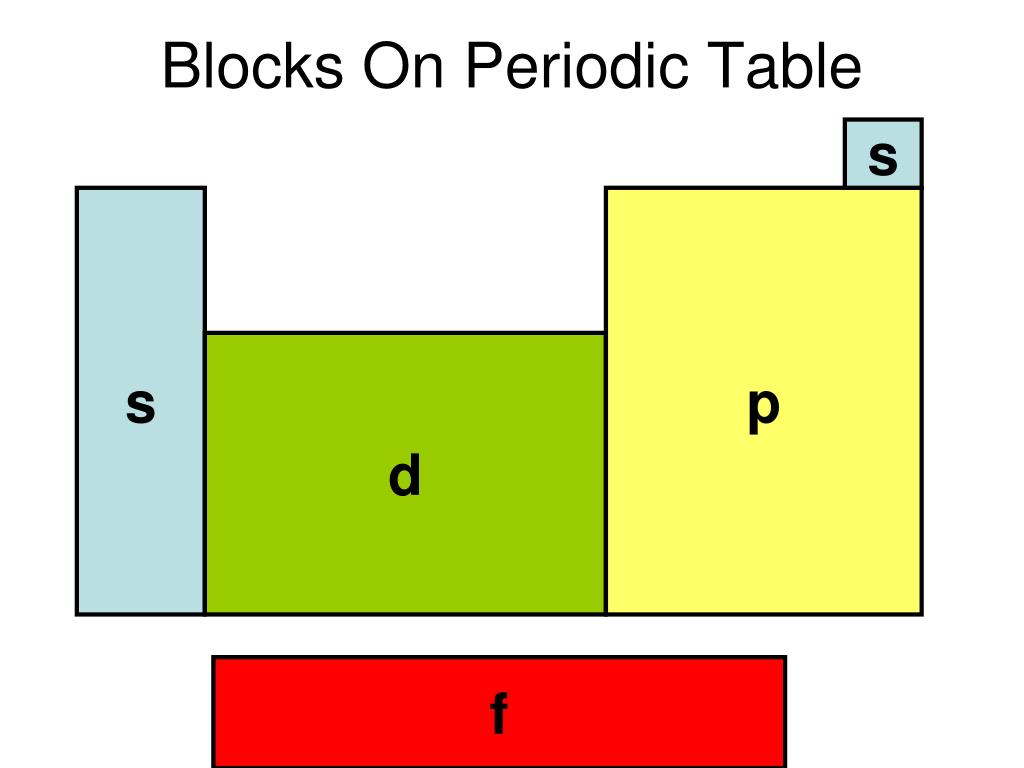
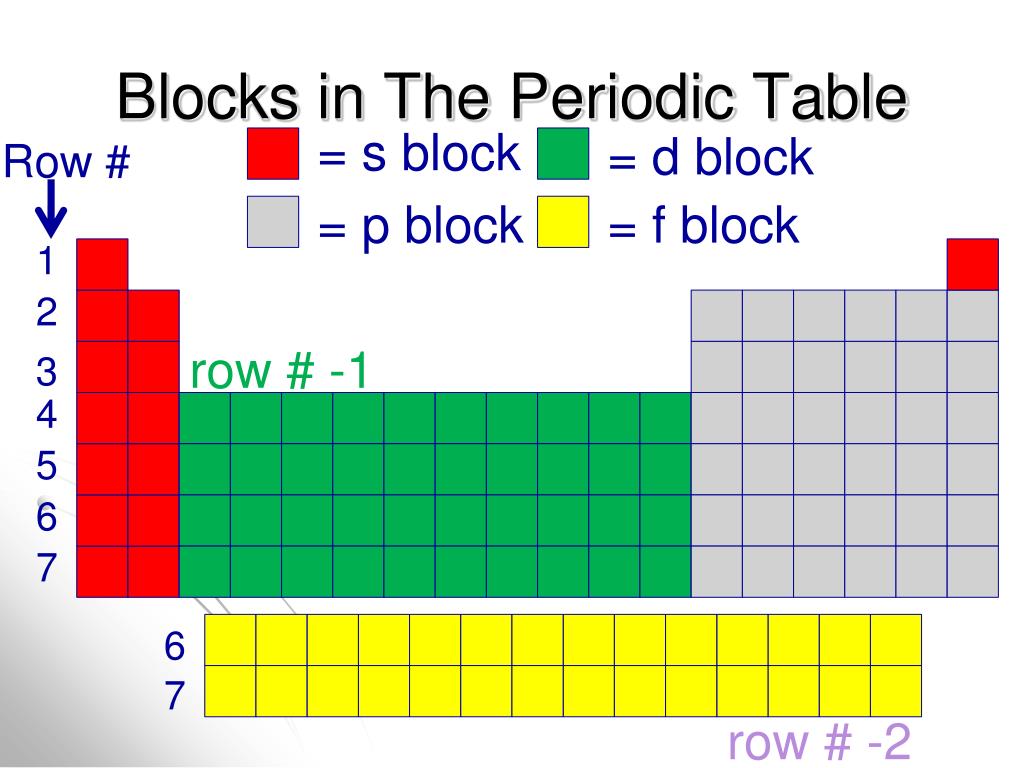
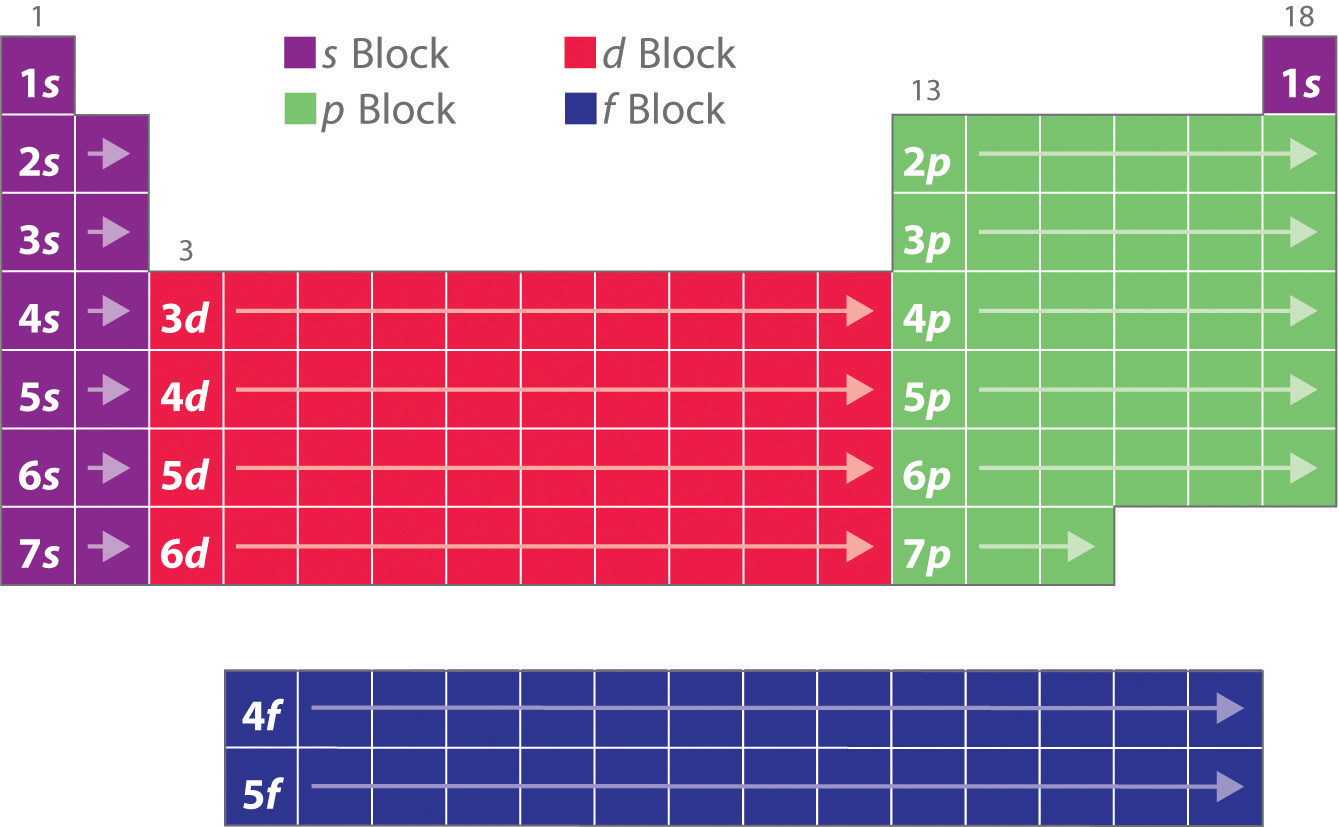
These sublayers being designated by the letters s, p, d, f or even g, the corresponding blocks are designated by these same letters. There are four blocks in the standard periodic table, plus a fifth appearing from the hypothetical 8th period: block s for ℓ = 0; block p for ℓ = 1; block d for ℓ = 2; block f for ℓ = 3;
Periodic Table Blocks S P D F Periodic Table Timeline
We can classify it into four blocks S - Block Elements. General Electronic configuration is ns 1-2; This block is situated at the extreme left of the periodic table and contains elements of group 1 and 2. Group I elements are known as alkali metals. Group II elements are known as Alkali earth metal. These elements are soft metals.

Representative Elements Definition, Examples, Diagrams
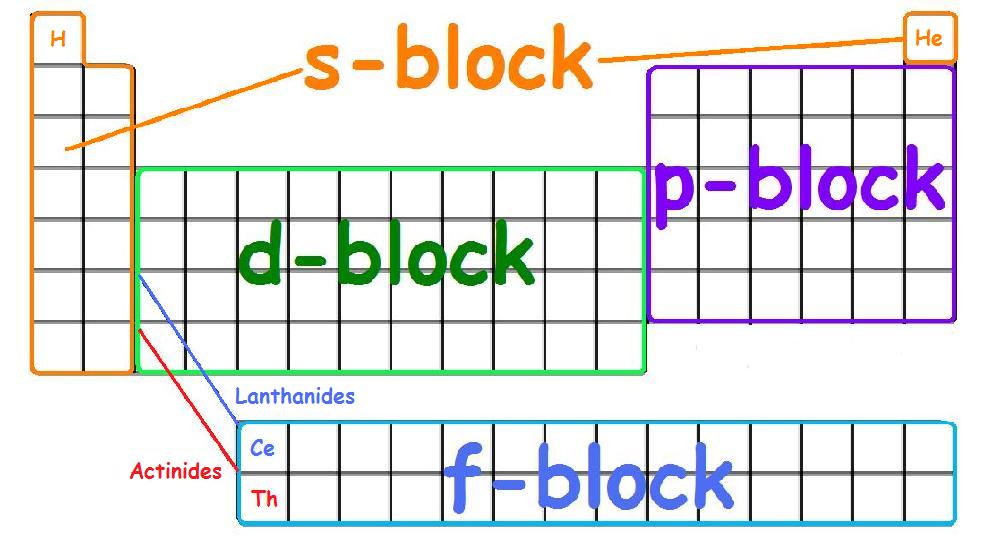
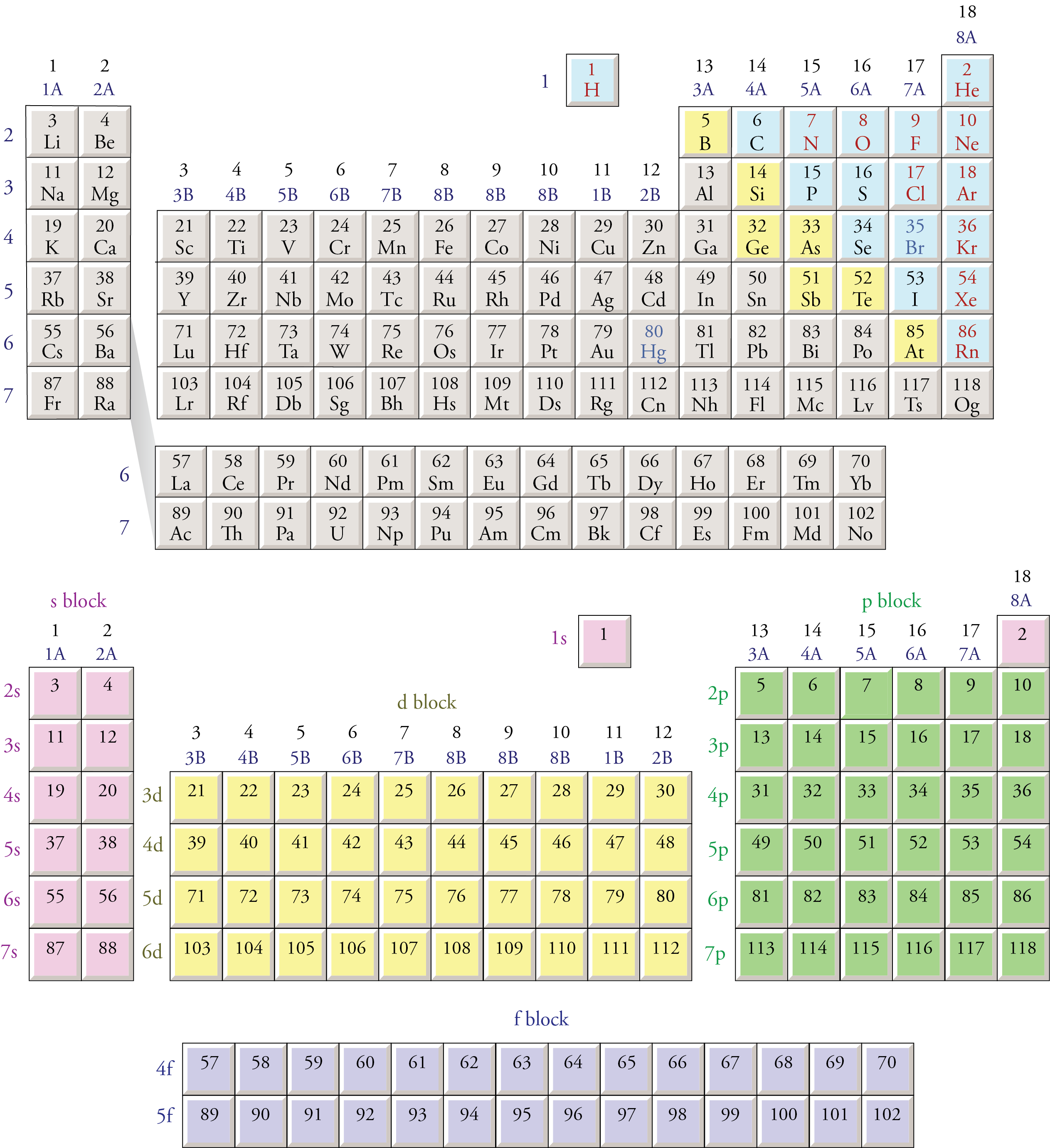
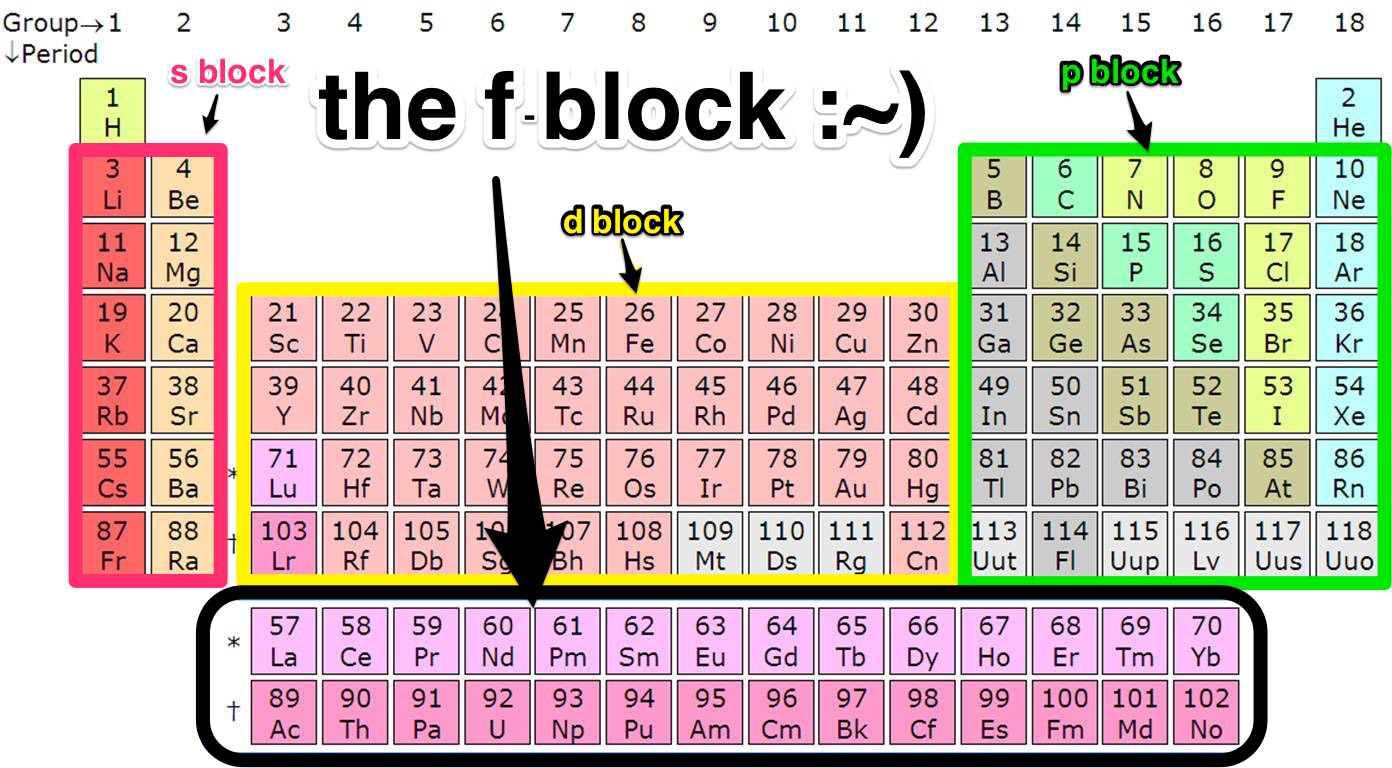
Each orbital can be represented by specific blocks on the periodic table. The s-block is the region of the alkali metals including helium (Groups 1 & 2), the d-block are the transition metals (Groups 3 to 12), the p-block are the main group elements from Groups 13 to 18, and the f-block are the lanthanides and actinides series.
Electron Configuration of Transition Metals Chemwiki
The two far-left columns of the periodic table constitute the s-block. For these elements, the last electron enters an s-orbital. Group one elements except for hydrogen are called the alkali metals and are extremely reactive as they have only one valence electron.. The d-block consists of the ten columns placed between the s and p-block.

Periodic Table Blocks S P D F
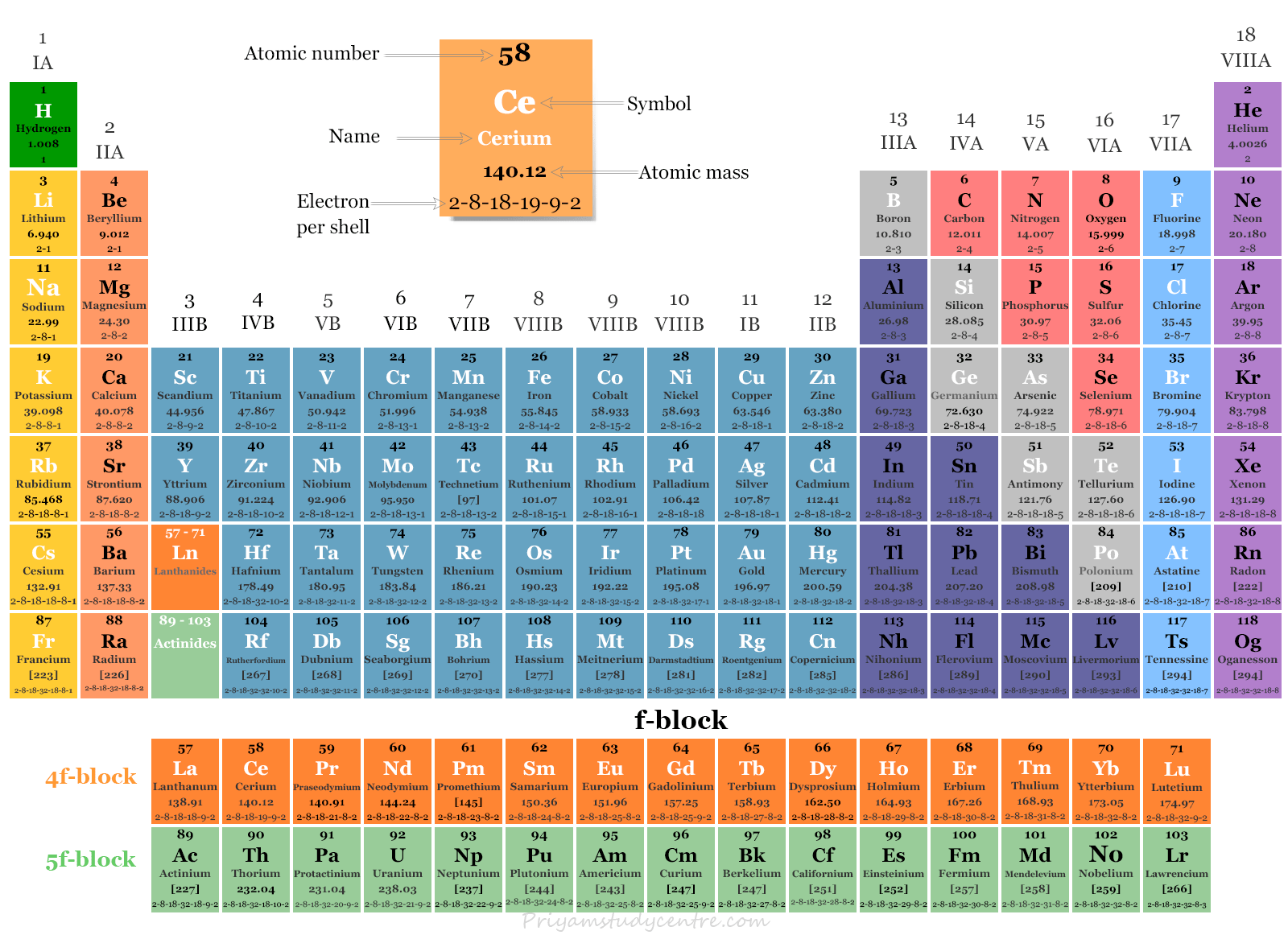
Properties of f-Block elements: (contain f-electrons in their valence shell). Electronic configuration: n s 2 (n − 1) d (0 − 1) (n − 2) f (1 − 14) 1) f - block elements are also called as inner transition elements. They are: Lanthanides and actinides. 2) Lanthanides. (58Ce- 71Lu ) 3) Actinides are the elements in which the last.

Download Periodic Table Of Elements S P D F Blocks Online Printable PDF DOC
The periodic table of elements can be organized by blocks (s, p, d, f, g). Learn what element blocks are and their properties and characteristics.. P-block: P-block elements include the last six element groups of the periodic table, excluding helium. The p-block elements include all of the nonmetals except for hydrogen and helium, the.
PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free download ID1755381
Download scientific diagram | The periodic table of s‐, p‐, d‐, and f‐block elements. from publication: The Pivotal Role of s‐, p‐, and f‐Block Metals in Water Electrolysis: Status.
PPT Blocks in The Periodic Table PowerPoint Presentation, free download ID2061175
In this article, we are going to study the similarities and periodicity of elements across the periodic table. We will study the different blocks: s-block, p-block, d-block, f-block and their characteristic properties, metals, metalloids, nonmetals, halogens, and noble gases. Read more about the Position of Hydrogen in Periodic Table, here.
Electron configurations
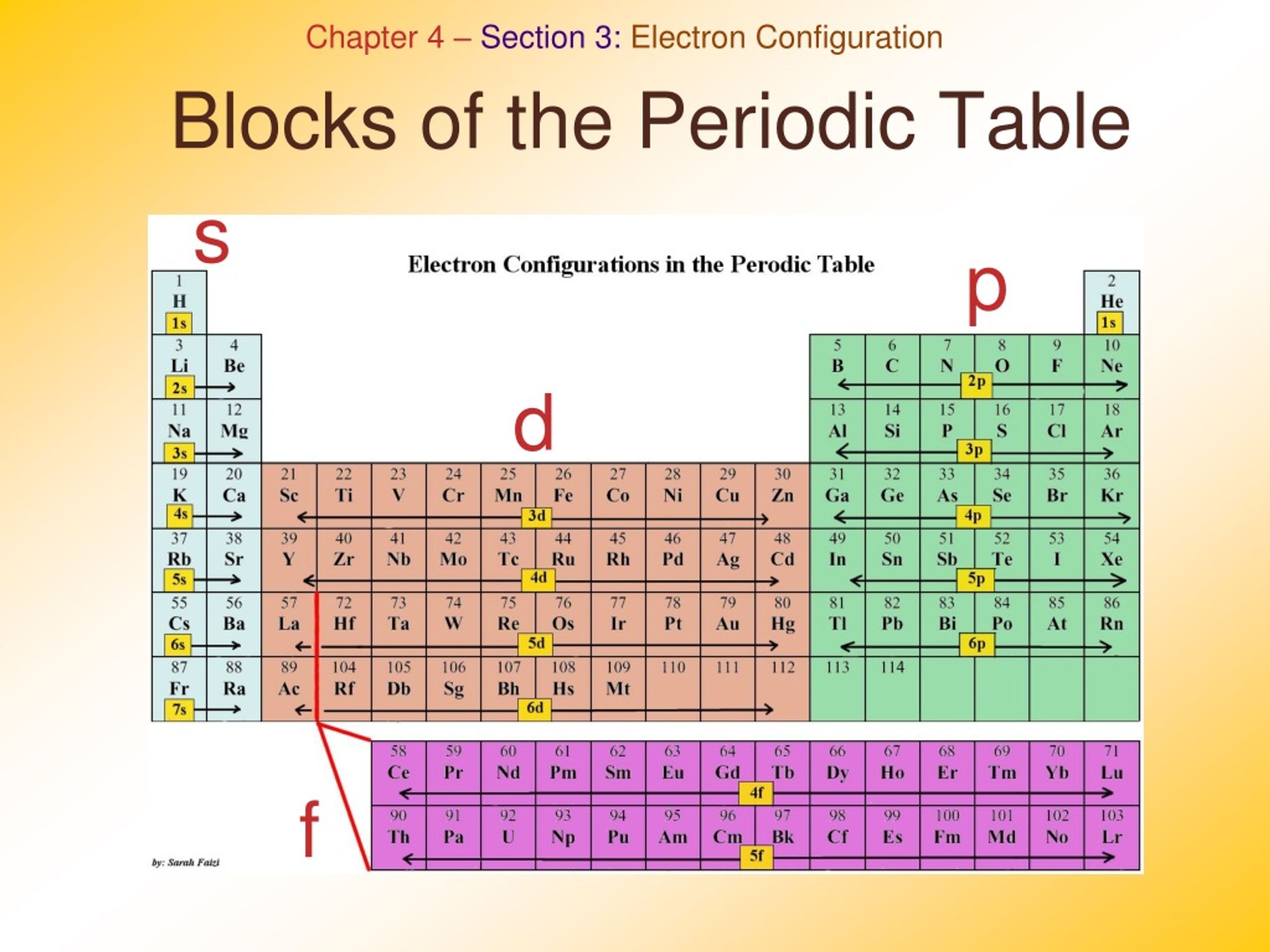
The element blocks are s, p, d, and f. They are determined by the valence electron orbital. Periodic table blocks are sets of elements grouped by their valence electron orbitals. The four block names are s-block, p-block, d-block, and f-block. Should a new element be discovered, it will be in g-block.

Modern Periodic Table s,p,d,f Blocks Elements Periodic table blocks, Periodic table, Element
A The group 2 elements are in the s block of the periodic table, and as group 2 elements, they all have two. np, nd, and nf orbitals to produce the distinctive chemical properties of the elements in the s block, p block, d block, and f block, respectively. 6.9: Electron Configurations and the Periodic Table is shared under a CC BY-NC-SA 4.

Periodic Table Of Elements With Orbitals
Periodic Table #1 | Introduction | Periods and Groupshttps://youtu.be/EZYtGgND1REClassification of elements and periodicity in properties #2 | Learning of Pe.
The FBlock An introduction Scienceline
Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The \(s\), \(p\), \(d\), and \(f\) blocks are illustrated below. Figure \(\PageIndex{2}\) (Credit: Christopher Auyeung and Joy Sheng; Source: CK-12 Foundation; License: CC BY-NC 3.0(opens in new window))
Periodic Table Blocks S P D F Periodic Table Timeline
Therefore, we can finally conclude that all the elements exist in their respective blocks, namely s block, p block, d block and f block elements. The S block contains the elements of Group 1 and 2 of the periodic table. D block elements are those elements that can be found in the modern periodic table from the third group to the twelfth group.

Periodic Table Blocks S P D F Periodic Table Timeline
The arrangement of atoms in the periodic table results in blocks corresponding to filling of the. np, nd, and nf orbitals to produce the distinctive chemical properties of the elements in the s block, p block, d block, and f block, respectively. 1.1.9: Electron Configurations and the Periodic Table is shared under a CC BY-NC-SA 4.0 license.
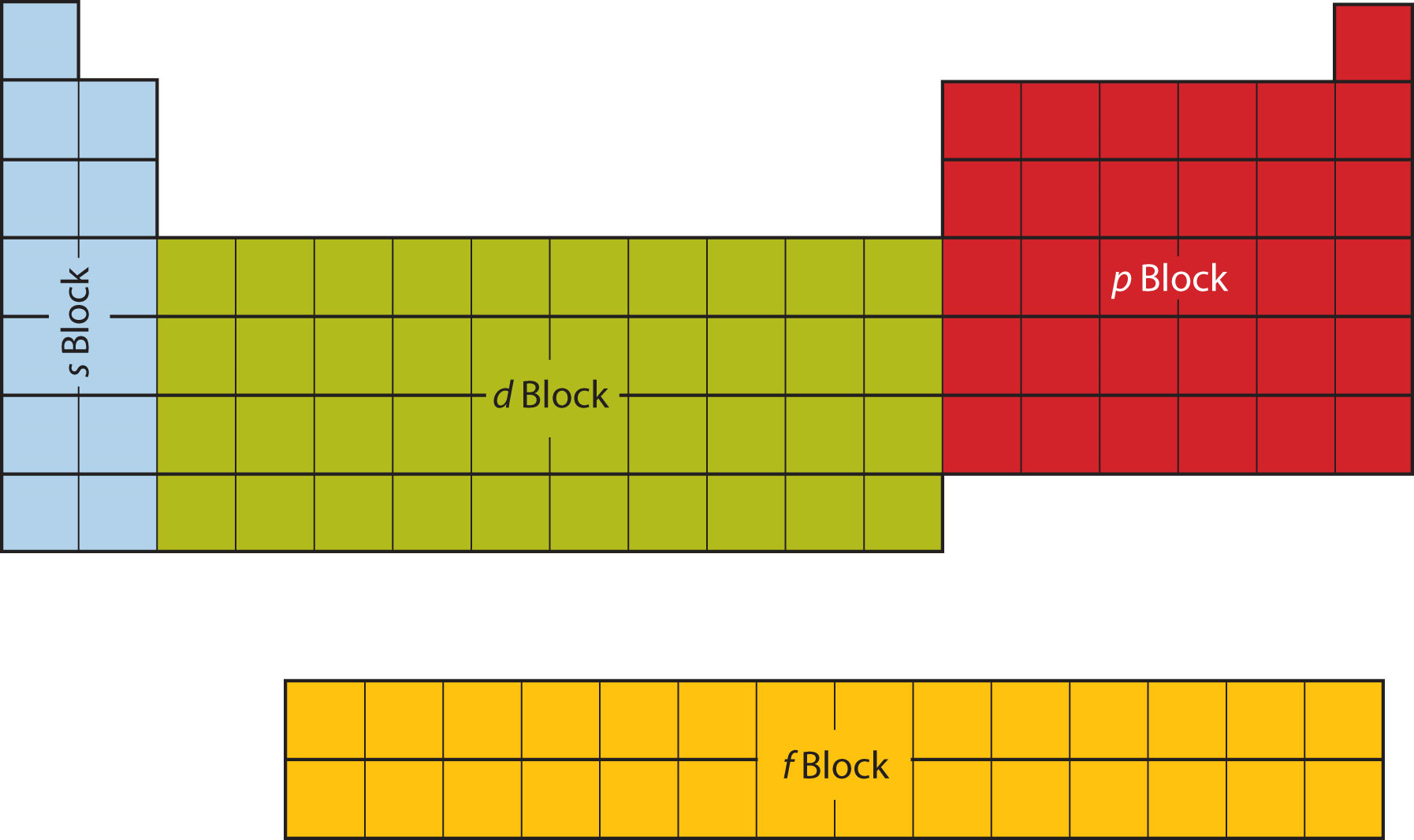
8.4 Electronic Structure and the Periodic Table Chemistry LibreTexts
The labels s, p, d and f blocks of the Periodic Table refer to the subshell that is being filled with electrons. ⚛ Group 1 elements occur at the beginning of a new row (Period) of the Periodic Table. The highest energy level (valence shell) contains only 1 electron in an s subshell. ⚛ Group 2 elements occur directly to the right of Group 1.